AHA科学声明:评估肾素-血管紧张素-醛固酮系统在母体和后代心血管健康产前规划中的作用的临床前证据
2025-08-25
999+
1.08MB
15 页
海报
侵权投诉
Hypertension
Hypertension. 2023;80:00–00. DOI: 10.1161/HYP.0000000000000227 TBD 2023 e1
AHA SCIENTIFIC STATEMENT
Appraising the Preclinical Evidence of the Role
of the Renin-Angiotensin-Aldosterone System
in Antenatal Programming of Maternal and
Offspring Cardiovascular Health Across the Life
Course: Moving the Field Forward: A Scientific
Statement From the American Heart Association
Barbara T. Alexander, PhD, FAHA, Chair; Andrew M. South, MD, Vice Chair; Phyllis August, MD; Mariane Bertagnolli, PhD;
Erin P. Ferranti, PhD; Justin L. Grobe, PhD; Emily J. Jones, PhD, FAHA; Analia S. Loria, PhD; Basmah Safdar, MD;
Maria Luisa Soledad Sequeira-Lopez, MD; on behalf of the American Heart Association Council on the Kidney in Cardiovascular
Disease; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Hyper-
tension; and Council on Lifestyle and Cardiometabolic Health
ABSTRACT: There is increasing interest in the long-term cardiovascular health of women with complicated pregnancies and
their affected offspring. Emerging antenatal risk factors such as preeclampsia appear to increase the risk of hypertension
and cardiovascular disease across the life course in both the offspring and women after pregnancy. However, the antenatal
programming mechanisms responsible are complex and incompletely understood, with roots in alterations in the development,
structure, and function of the kidney, heart, vasculature, and brain. The renin-angiotensin-aldosterone system is a major
regulator of maternal-fetal health through the placental interface, as well as kidney and cardiovascular tissue development
and function. Renin-angiotensin-aldosterone system dysregulation plays a critical role in the development of pregnancy
complications such as preeclampsia and programming of long-term adverse cardiovascular health in both the mother and
the offspring. An improved understanding of antenatal renin-angiotensin-aldosterone system programming is crucial to
identify at-risk individuals and to facilitate development of novel therapies to prevent and treat disease across the life
course. Given the inherent complexities of the renin-angiotensin-aldosterone system, it is imperative that preclinical and
translational research studies adhere to best practices to accurately and rigorously measure components of the renin-
angiotensin-aldosterone system. This comprehensive synthesis of preclinical and translational scientific evidence of the
mechanistic role of the renin-angiotensin-aldosterone system in antenatal programming of hypertension and cardiovascular
disease will help (1) to ensure that future research uses best research practices, (2) to identify pressing needs, and (3) to
guide future investigations to maximize potential outcomes. This will facilitate more rapid and efficient translation to clinical
care and improve health outcomes.
Key Words: AHA Scientific Statements ◼ aldosterone ◼ angiotensin-converting enzyme 2 ◼ hypertension ◼ pre-eclampsia ◼ pregnancy
◼ renin-angiotensin system
The antenatal period, spanning conception to birth,
is critical for maternal and fetal health. Exposure
to adverse health conditions and environmental
stressors during this time period can have long-term
consequences on the mother and her offspring. Briefly
stated, antenatal programming happens when expo-
sures occur from conception through birth that alter
structural, physiological, and metabolic fetal devel-
opment and maternal health to improve short-term
survival but at the expense of programmed adverse
© 2023 American Heart Association, Inc.
Hypertension is available at www.ahajournals.org/journal/hyp
Downloaded from http://ahajournals.org by on March 23, 2023
CLINICAL STATEMENTS
AND GUIDELINES
e2 TBD 2023 Hypertension. 2023;80:00–00. DOI: 10.1161/HYP.0000000000000227
Alexander et al Antenatal Programming of Cardiovascular Health
cardiovascular health in the long term (ie, developmen-
tal plasticity).1 For example, maternal hypertension, the
most common medical comorbidity in pregnancy, is a
major health concern and is associated with increased
risks of short-term mortality and morbidity, as well as
programmed chronic disease later in life in both the
mother and the fetus.2 Offspring of women with pre-
eclampsia have lower birth weight and higher blood
pressure throughout childhood and young adulthood
compared with unexposed offspring.3 Numerous pre-
clinical models have confirmed this association,4 yet
the exact mechanisms remain incompletely understood.
Several of the major components of the renin-angio-
tensin-aldosterone system (RAAS) regulate several
key physiological processes in both mother and fetus
during pregnancy and the development and function
of the kidney and cardiovascular system. Most nota-
bly, these include the angiotensin-converting enzyme
(ACE)/angiotensin II (Ang II)/Ang II type 1 receptor
(AT1R) and the ACE2/angiotensin-(1–7) (Ang-[1–7])/
Mas receptor pathways. Dysregulation of the circulat-
ing and tissue-specific RAAS contributes to the patho-
genesis of numerous antenatal conditions, including
hypertensive disorders in pregnancy.5 RAAS dysregu-
lation is one potential mechanism for the long-term
programming of hypertension in offspring exposed to
preeclampsia and other adverse antenatal factors.4,6
Greater risk of long-term hypertension and cardio-
vascular disease is also observed in women after pre-
eclampsia, highlighting that the burden of programmed
cardiovascular disease is not limited to the offspring.7 It
is important to note that, despite decades of research,
recommendations for preeclampsia treatment have not
changed,8 and the prevalence of hypertension in preg-
nancy continues to increase.9 Treatment strategies vary
considerably around the world, with significant dispari-
ties in the screening and follow-up for the development
of hypertension and cardiovascular disease in affected
women during the postpartum period and beyond, includ-
ing in women from disenfranchised populations in the
United States.10 In addition, emerging evidence indicates
that pregnancy may place women at greater risk for
severe acute respiratory syndrome coronavirus 2 (SARS-
CoV-2) infection, which in turn may increase the risk of
several pregnancy complications, including preeclampsia
and low birth weight, possibly related to altered ACE2
expression, the binding site for SARS-CoV-2.11,12 Thus,
the short- and long-term increased risk of hypertension
and cardiovascular disease in the mother and offspring
attributable to pregnancy complications remains a critical
health concern.
Despite decades of high-quality studies that have
provided insights into the mechanisms responsible for
the programming of cardiovascular disease associated
with complicated pregnancies, there remains a crucial
need in the field to further characterize dysregulatory
events affecting the RAAS during this critical period
of life. Primary reasons for persistent knowledge gaps
include heterogeneity in the methods used in many pre-
clinical models and the complex nature of the RAAS that
makes accurate and reliable quantification challenging.
Thus, the goal of this scientific statement is to summa-
rize the current state of knowledge related to preclini-
cal evidence of antenatal programming mechanisms of
long-term maternal and offspring cardiovascular health
as it relates to the role of several of the major RAAS
pathways using well-characterized preclinical models
of developmental programming. This scientific state-
ment identifies gaps in knowledge that require further
research. Moreover, this scientific statement empha-
sizes the importance of better understanding program-
ming mechanisms for both investigators and clinicians
to develop targeted interventions to prevent or mitigate
the increased risk of hypertension and cardiovascular
disease. This is the first step in an approach to reduce
future cardiovascular risk in women with complicated
pregnancies and their children.
A comprehensive literature search was conducted
from approximately September 15, 2021, to Novem-
ber 15, 2021, that encompassed preclinical and clini-
cal studies and reviews that were published in PubMed,
Scopus, and other relevant databases using standardized
methods. Key search words included but were not lim-
ited to pregnancy, preeclampsia, RAAS, high blood pres-
sure, hypertension, cardiovascular, renal, brain, placental
insufficiency, hypoxia, glucocorticoids, maternal under-
nutrition, offspring, and chronic health. The selection of
writing group members was based on a wide range of
expertise, including clinical and preclinical researchers
representing different backgrounds, geographic regions,
sexes, races, and ethnicities.
MATERNAL-PLACENTAL-FETAL INTERFACE
AND THE RAAS
Maternal Cardiovascular Physiology During
Pregnancy
Maternal cardiovascular and renal adaptations to preg-
nancy are essential to accommodate the physiologi-
cal stress imparted by the growing fetus and placenta.
Marked systemic vasodilation with decreased systemic
vascular resistance and subsequent lower blood pres-
sure characterizes early pregnancy starting at 4 to 6
weeks of gestation. This likely stimulates the mater-
nal circulating RAAS by the end of the first trimester
to retain sodium and fluid to increase plasma volume
progressively throughout gestation, up to 40% to 50%
higher than the prepregnancy baseline.13 Cardiac output,
renal blood flow, and glomerular filtration rate increase to
≈50% over baseline; these changes are apparent by the
second trimester and persist until term.
Downloaded from http://ahajournals.org by on March 23, 2023
CLINICAL STATEMENTS
AND GUIDELINES
Hypertension. 2023;80:00–00. DOI: 10.1161/HYP.0000000000000227 TBD 2023 e3
Alexander et al Antenatal Programming of Cardiovascular Health
The RAAS in Normal Pregnancy Physiology
The RAAS, a crucial regulator of blood pressure and
fluid-electrolyte balance, particularly in pregnant women
and the fetus, is a key contributor to cardiovascular and
kidney development (Figure 1A). Assessment of RAAS
components in the maternal circulation during pregnancy
suggests that overall activation that contributes to the
aforementioned physiological cardiovascular changes.
In normotensive, healthy pregnant women, blood pres-
sure remains lower while plasma renin activity (PRA) and
aldosterone remain elevated until late in pregnancy when
blood pressure increases.14 Increased angiotensinogen
production and PRA lead to increased angiotensin I
concentrations, favoring augmented Ang II production
that occurs despite reduced serum ACE activity, in part
as a result of activation of additional RAAS pathways.15
Ang II–mediated increased aldosterone concentrations
directly stimulate renal sodium and fluid retention to
increase blood volume.
Circulating and local tissue Ang II exerts key physi-
ological functions in many crucial steps of placentation,
including trophoblast invasion and migration, as well as
spiral artery remodeling.16 RAAS components show a
dynamic distribution throughout pregnancy. The AT1R is
expressed in trophoblasts in early pregnancy but also
in villous endothelial cells at term.17 Prorenin, (pro)renin
receptor, AT1R, and Ang II type 2 receptor proteins are
also expressed throughout gestation in trophoblasts
at the maternal-fetal interface and in invasive tropho-
blasts, whereas ACE is concentrated predominantly in
the fetal circulation, particularly in endothelial cells.18
The incremental ACE protein expression in fetal endo-
thelial cells throughout pregnancy favors enhanced Ang
II production in placental vessels from the fetal side,
where angiogenesis, an essential process for maintain-
ing fetal perfusion, continuously occurs.18 However, the
expected increased Ang II production in the fetus and
mother must be finely modulated to prevent excessive
vasoconstriction and cardiovascular remodeling that
could occur if Ang II concentrations increase above the
expected physiological range.19
Pregnancy also stimulates the ACE2/Ang-(1–7)
pathway to balance increased ACE/Ang II pathway
activity and to contribute to maternal hemodynamic
adaptations and placentation, trophoblast invasion,
decidualization, and vascular remodeling.15,20 ACE2
breaks down Ang II, and Ang-(1–7), acting on its Mas
receptor, antagonizes Ang II signaling through AT1R
modulation.6 Estrogens regulate the progressive RAAS
activation observed throughout gestation in part by
directly stimulating angiotensinogen production and
increasing ACE2 expression and activity in local tis-
sue.21 In rats, renal ACE2 and Ang-(1–7) are progres-
sively upregulated throughout pregnancy.22 ACE2 and
Ang-(1–7) are also expressed in trophoblasts, villous
vessel endothelial cells, primary villi vascular smooth
muscle cells, and the syncytium and decidua.23 ACE2/
Ang-(1–7) expression and activity in the placenta are
dynamic, with greater concentrations in the decidua in
early pregnancy that progressively change toward the
placental villous endothelial cells and trophoblasts in
late gestation.23 The presence of the ACE2/Ang-(1–7)
pathway in invasive trophoblasts surrounding the spi-
ral arteries, as well as in endothelial cells and vascular
smooth muscle cells, suggests that the ACE2/Ang-
(1–7) pathway helps regulate uterine artery tone and
reduce maternal systemic vascular resistance.15 There-
fore, ACE2-mediated conversion of Ang II into Ang-(1–
7) and intracellular signaling between AT1R and Mas
receptor are likely key factors regulating Ang II physi-
ological effects during pregnancy.
Additional RAAS pathways contribute to the Ang II–
Ang-(1–7) balance during pregnancy but are less well
characterized; thus, their role in antenatal programming
presents another important knowledge gap. The (pro)
renin receptor is crucial for many developmental and
physiological processes during pregnancy through sev-
eral signaling pathways, including Wnt/b-catenin and
mitogen-activated protein kinase.24 Neprilysin, another
metallopeptidase that converts angiotensin I to Ang-(1–
7), has unclear effects during pregnancy. Compared with
pregnant women with healthy weight, pregnant women
with overweight or obesity (body mass index ≥25 or ≥30
kg/m2, respectively) have lower endothelial cell neprilysin
expression in the fetus and placenta, and fetal weight
is associated inversely with circulating neprilysin levels
in cord blood.25 Uterine mast cell and natural killer cell
secretion of chymase, a serine protease that generates
Ang II independently of ACE, may contribute to decidual
vessel remodeling and subsequent fetal growth.26
The RAAS in Pregnancy Pathologies
Placental insufficiency is a hallmark of many adverse
pregnancy events that program later disease in the
offspring. Short- or long-term interruptions in the suffi-
cient delivery of blood, oxygen, or nutrients to the fetus
can alter fetal growth and organ development, leading
to abnormal tissue structure and function, especially in
the kidneys. Adequate fetal perfusion requires sufficient
maternal cardiovascular and placental health; hence,
interruptions in the maternal, placental, or fetal RAAS
can potentially adversely affect fetal and maternal car-
diovascular health in the short and long term (Figure 1B).
Human intrauterine growth restriction, a proxy for pla-
cental insufficiency, is associated with higher cord blood
Ang II concentration but no difference in fetal-placental
AT1R concentration compared with term pregnancies
with delivery by elective cesarean section.27 Uterine ves-
sel ligation or clamping during mid or late pregnancy
as a model of placental insufficiency and maternal
Downloaded from http://ahajournals.org by on March 23, 2023
摘要:
展开>>
收起<<
Hypertension.2023;80:00–00.DOI:10.1161/HYP.0000000000000227TBD2023e1AHASCIENTIFICSTATEMENTAppraisingthePreclinicalEvidenceoftheRoleoftheRenin-Angiotensin-AldosteroneSysteminAntenatalProgrammingofMaternalandOffspringCardiovascularHealthAcrosstheLifeCourse:MovingtheFieldForward:AScientificStatementFro...
相关推荐
-
妇科诊疗技术操作标准规范_20622084

 2025-11-29 999+
2025-11-29 999+ -
妇科诊疗常规(已审)_20625126

 2025-11-29 999+
2025-11-29 999+ -
妇科人流综合症应急预案及流程_20622076
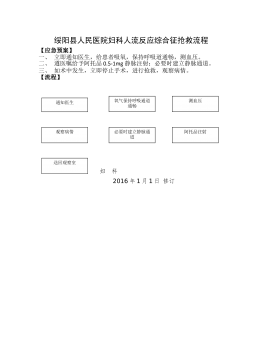
 2025-11-29 999+
2025-11-29 999+ -
妇科检查及二合诊三合诊图解_20622068

 2025-11-29 999+
2025-11-29 999+ -
妇科检查规范_20622067

 2025-11-29 999+
2025-11-29 999+ -
妇科护理常规讲解_20625121

 2025-11-29 999+
2025-11-29 999+ -
妇科护理常规_20622061

 2025-11-29 999+
2025-11-29 999+ -
妇科 不孕病(多囊卵巢综合征)中医临床路径_20625117

 2025-11-29 999+
2025-11-29 999+ -
二级病原微生物试验室生物安全管理规范-上海卫生和计划生育_20621748

 2025-11-29 999+
2025-11-29 999+ -
2022子宫内膜异位症【29页】

 2025-11-29 999+
2025-11-29 999+



